/science---at-work-in-the-lab-183295497-56f17d453df78ce5f83c036a.jpg) The acid dissociation constant, Ka, measures how strong an acid is in a solution. Ka helps us identify if an acid is strong or weak based on its dissociation level.
The acid dissociation constant, Ka, measures how strong an acid is in a solution. Ka helps us identify if an acid is strong or weak based on its dissociation level. In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid–base reactions.
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid–base reactions.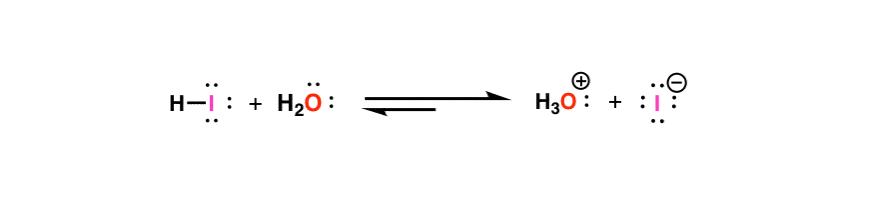 The acidity constant Ka is the equilibrium constant for dissociation of an acid into its conjugate base and H+. Pka is the negative logarithm of Ka.
The acidity constant Ka is the equilibrium constant for dissociation of an acid into its conjugate base and H+. Pka is the negative logarithm of Ka.